At Arc Nouvel,
we partner with clients to accelerate innovation and bring transformative therapies to patients worldwide.
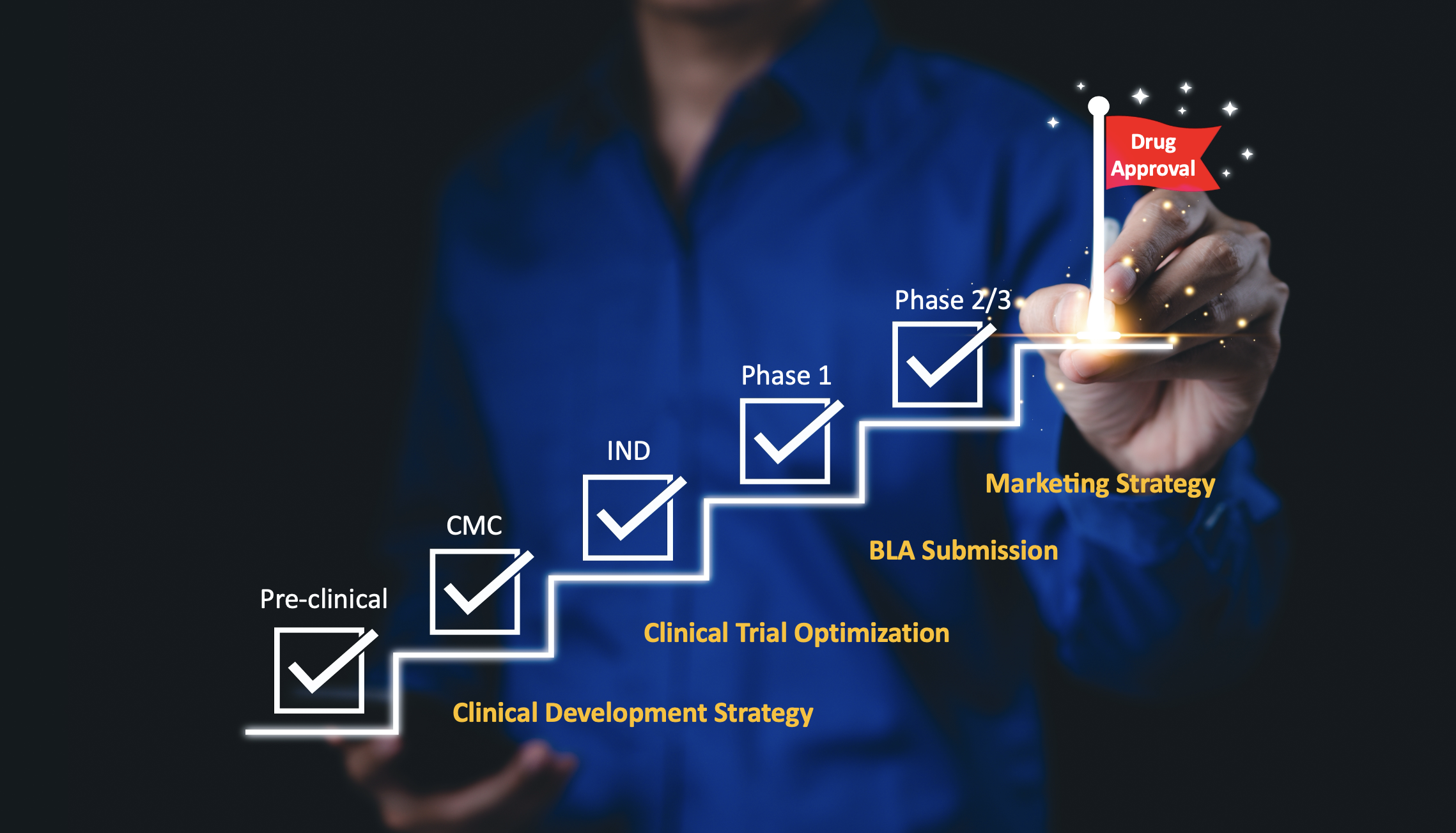
Our expertise spans the full spectrum of clinical development, regulatory strategy, and organizational growth.
Comprehensive CMC Strategy
We craft integrated Chemistry, Manufacturing, and Controls (CMC) strategies that include portfolio prioritization, manufacturing design and capacity planning supported by quantitative modeling, and resilient supply chain strategies—helping you streamline development, reduce risk, and accelerate time to market.
Competitive Landscape Assessment
We conduct in-depth assessments of the global competitive landscape to inform strategic decision-making. This includes evaluating therapeutic indications, market size potential, and competitive dynamics to shape clinical development strategies that are both scientifically sound and commercially viable.
Global Clinical Trial Leadership & Operations (Phase I–IV)
We provide proactive leadership and operational management for all aspects of clinical trial execution across Phase I–IV clinical trials. Our proactive approach addresses key challenges such as patient enrollment, accelerated timelines, and operational efficiency. Services include global site selection, study start-up readiness, CRO and vendor selection and oversight, and comprehensive medical monitoring support—encompassing safety surveillance, data review, and efficacy assessments to ensure trial integrity and success.
Global Regulatory Support
We navigate the complexities of global regulatory frameworks to accelerate submissions and approvals, providing expertise across IND filings to enable global submissions, Biologics License Applications (BLAs) with agencies such as the FDA, EMA, PMDA, and NMPA, and comprehensive guidance for addressing regulatory questions, requests, and feedback. Our support extends to regulatory agency meeting preparation, documentation, and attendance—whether live or virtual—ensuring a seamless and effective pathway to approval.
Coaching & Leadership Development
Biomarker & Diagnostic Testing Strategy
We design and advance biomarker strategies that span identification, validation, and companion diagnostic (CDx) readiness. Our integrated approach enables precision medicine initiatives, reduces risk, and accelerates the path to regulatory approval.
Quality Assurance, Compliance & Inspection Readiness
We strengthen quality systems and ensure alignment with global regulatory standards. Our team guides organizations in building compliance frameworks, mitigating risk, and preparing for inspections—positioning you for successful audits and long-term operational excellence.
Scientific Advice Meeting Preparation & Support
We prepare organizations for regulatory advisory and scientific advice meetings. Services include development of meeting materials, readiness assessments, and participation in both mock sessions and live interactions with global regulators.
Data Interpretation & Strategic Insights
We provide expert data interpretation and analysis from pre-clinical through late-stage clinical development to guide your next steps with confidence. Our approach includes rigorous endpoint evaluation and the integration of input from global thought leaders, ensuring development programs are scientifically robust, clinically meaningful, and aligned for regulatory and commercial success.
US Subsidiary Setup & Market Entry Support
We guide overseas biopharmaceutical and life sciences companies in establishing a strong presence in the United States. Our support includes legal and operational structuring of US subsidiaries, navigation of regulatory and compliance requirements, and strategic planning for clinical development within the US landscape. We also advise on talent acquisition, vendor and CRO partnerships, and market entry strategies—helping international organizations accelerate their US expansion while minimizing risk and ensuring long-term success.



